As i expected, pALA1029, pOG04 DRW and pOG4003 have once again shown no growth, almost in sympathy the serial dilution i performed yesterday yielded poor results. The spread plates only showed colony growths of 1-14, as i was looking for colonies around 100, i decided that it was not worth replica plating. I have still carried out a serial dilution from the overnight broth cultures and spread plated them onto LB agar plates and left them to incubate at 37C overnight. I do not expect any growth based on todays results but one can hope!
Category: Uncategorized
Another frustrating day in the lab Grew pNC0412B…
Another frustrating day in the lab!!
Grew pNC0412B to an OD of 1 then performed a serial dilution (A,B,C,D -1to-5.5), I innoculated a fresh 10ml LB broth with 100 microlitres from dilution -4 and placed into the 37C shaker overnight. I then spread plated 100 microlitres from dilution -5 onto a fresh LB agar plate, I also spread plated 100 microlitres from dilution -5.5 onto a fresh LB agar plate, then placed these into the 37C incubator overnight. I would have also done this for pOG04 NJC but it refused to grow, after 6 hours in the 37C shaker only an OD of 0.01 was achieved, i abandoned this set of broths. The plates i hoped to grow for today containing the plasmids pALA1029, pOG4003 and pOG04 DRW also showed no growth. I have tried again today so hopefully tomorrow will be a better day. I seriously think C2110 is the work of the devil!
Hello Today was our last day in the…
Hello!
Today was our last day in the lab! 🙁 We filmed our latest and final videoblog, removed the gel, stained and viewed it. We saw some separation from all but one of the plugs, however separation was very blurred. We decided to run the same samples again, but for longer and with a higher switch interval. We set up and ran a new gel for 18 hours, at 6V/cm, with a 1-30 second switch interval, a 1.4% gel with modified buffer flow.
Mike will remove the gel tomorrow, stain it and view for us. Hopefully we will see better separation, from the samples containing enough DNA.
The last 10 weeks have been amazing – i have learnt so much since i started and it’s going to be really odd to not be in the lab everyday. I have really enjoyed working through a project, having to work on problems and figure out solutions. Then getting to see the good and bad results. I am very impressed with myself, as i set out to blog everyday i was in the lab with what i have done, the good and the bad! and i have actually managed it! 🙂 I wasn’t sure about whether i would get on with videoblogging at the beginning, but i have really enjoyed filming them every week – although most the content was never included as we ALWAYS got distracted! I really hope those who have watched them have found them as interesting and enjoyable as we did.
It has been a very rewarding experience, and i am so grateful to everyone who has made the last 10 weeks, the best summer – you all know who you are! And of course Mike, who has put up with us and our random chatter, better than i ever imagined he would. It has been an absolute pleasure to work along side him, and i hope i get the chance to do it again.
Today may be Friday but because of the…
Today may be Friday but because of the bank holiday monday, i am treating today like a monday!!
I am growing two batches of C2110 up to an OD of 0.35. Then i will perform cell competancy and transformation. Normally i would put the spread plates in the 37C incubator overnight but because of the bank holiday i am placing the plates in the 25C until tuesday. Hopefully there will be some growth. If all goes well Tuesday i will be working with pNC0412B, pOG04NJC, pALA1029 and pOG4003.
Hey All Removed plugs from SpeI enzyme buffer…
Hey All,
Removed plugs from SpeI enzyme, buffer and BSA, and washed in 1x wash buffer for 30mins. We removed this, then incubated them in 0.5x TAE buffer for an hour, while we prepared a 1.4% agarose gel. We then loaded 1/2 of each plug strain into individual wells, as well as the low range PFG marker, sealed with agarose and ran at 6V/cm, for 15 hours, with a 1-12 sec switch interval, and modified buffer flow.
Tomorrow, we will remove the gel, stain it and view it. We will also be filming our last videoblog! 🙁 as it will be the last day of our project – 10 weeks has gone by sooo fast! We may also be running another gel, depending on the results of the first. See you all then 🙂
Had growth from two of my plasmids pOG04…
Had growth from two of my plasmids, pOG04 DRW and pNC0412B but nothing from pOG04 NJC. I used an original sample of pNC0412B and so i am very happy that there is growth, this means that there is probably a fault with my plasmid prep. At least i have something to work with. The pOG04 NJC is a known temperemental plasmid so no growth is not wholly unexpected, just frustrating. Today just counted my T end plates and recorded the data, the rest of the day is spent making up agars and broths ready for next week as it is the last week in the lab and i would like to be prepared!
Hello Today we removed the plugs from proteinase…
Hello,
Today we removed the plugs from proteinase K, and washed them in 1 x wash buffer 4 times, for an hour each. We then incubated them in 0.1 x wash buffer for an hour, then in 1 x SpeI buffer to saturate for an hour. We then removed and added fresh SpeI buffer, SpeI enzyme and BSA and incubated overnight at 37C. We also made up 500ml of 0.5x TAE buffer and autoclaved it for tomorrow.
Unfortunately, we lost a few plugs along the way, due to instability. Tomorrow we will be incubating the plugs in 1 x wash buffer, then 0.5x TAE, before running the new plugs.
Today i counted my T0 plates and replica…
Today i counted my T0 plates and replica plated T end plates, I have tried again with pNC0412B, pOG04 NJC and pOG04 DRW. Hopefully tomorrow there will be growth as i have used the original plasmid preps. Time to finish up with the maths and percentages as there is only 1 week left to go!!
Hi all counted yesterdays plates and replica plated…
Hi all,
counted yesterdays plates and replica plated pOG4 onto LB and LB containing amp100, then i counted and replica plated pnc0412B onto LB and LB containing chl20, then i incubated them overnight at 37C. My next task was to complete the second serial dilution and spread plated 10-5.5 and 10-6 onto LB agar plates with no antibiotics. Next i checked on pOG04NJC and pnc0412B – NO GROWTH!!! I am sure they know its monday! Having Checked with Ross williams he thinks it could be the plasmid at fault. Tomorrow i try again.
Hello All Unfortunately the re cultured samples hadn’t…
Hello All,
Unfortunately the re cultured samples hadn’t grown enough on Monday to use, so we left them another day and night until Tuesday. Today, although the growth was minimal we decided to start plug making. We added chloramphenicol to the samples, left them for an hour, and then centrifuged them all down until we had appropriate sized pellets. However, some of the samples did not readily form pellets, and several pellets were much smaller than others or were very dark in colour.
We then combined the pellets with cell suspension buffer, shook them to combine and added agarose. We then pipetted the samples into plug molds and left them to solidify in the fridge. Once solid, we pushed each plug into a separate eppendorf, added appropriate lysozyme, buffer and mutanolysin. However one plug hadn’t soldified enough and was discared. They are now incubating at 37C. At 6, i will remove the lysozyme, buffer and mutanolysin, and add proteinase K and buffer. Incubating them overnight at 50C.
Tomorrow we will be washing the plugs, and incubating them in restriction enzyme, buffer and BSA.
Today has been an almost perfect day in…
Today has been an almost perfect day in the lab.
pOG4 and pNC0412B have grown quite nicely to an OD of 1 (0.8-1), I have performed a serial dilution on both and left the innoculated broths overnight in the shaker at 37C, the spread plates of 5 & 5.5 are in the 37C incubator. Also today i innoculated another flask of LB broth with C2110 and grew to an OD of 0.35. Then i carried out cell competancy then transformed them, using the plasmids pOG04 NJC and pNC0412B. I spread plated pOG04NJC onto an LB agar plate containing amp50 and spread plated pNC0412B onto an LB agar plated containing chl10. these were then placed in the 37C incubator overnight. Busy day tomorrow as agars, broths, velvets and NaCl need to be made-up/autoclaved, as well as tomorrows tasks. Early start i think!
Hi all Counted the t end plates from…
Hi all,
Counted the t end plates from yesterday and recorded the results, will spend the weekend doing some scary maths. My streak plate of C2110 grew nicely so i now have a fresh sample to work with on monday. And FINALLY i have managed to get some growth from pNC0412B so i have refridgerated the plate and will be working with the chloramphenical resistant plasmid on monday, pOG4 has shown a little growth so i have placed it in the 25C incubater. Hopefully enough will have grown by monday so that i can complete the whole set of plasmids i was given to test. Am really looking forward to monday.
Hi Had a good couple of days have…
Hi,
Had a good couple of days, have been having trouble with growth – not yesterday each plate had 500+ colonies, think i need glasses now!! Today was an easy day too, counted yesterdays T end plates and recorded the results, then counted the plates from yesterdays serial dilution T0 plates and replica plated the ones with around 100 colonies, once again there was good growth so i replica plated ones with around 200 colonies.
Hello Everyone Not much to tell today our…
Hello Everyone,
Not much to tell today, our new plugs contained far too much DNA and cells, and therefore were very delicate and almost all broke apart during the removal of proteinase K, and addition of 1x wash buffer this morning. We decided to stop production of these plugs, and begin again next Monday as plug making takes around 3 whole days in a row. Hopefully we can perfect the amount of DNA and cells present in the new plugs, to avoid this problem, through measurement of OD/cell density after addition of cell suspension buffer after centifuge.
I will be carrying on writing my final report for the project for the next 2 days, instead of being in the lab and start fresh with lab work and plug making on Monday! See you all then 🙂
Hey All When we checked the growth of…
Hey All,
When we checked the growth of the 10 different strain samples of propionibacterium acnes this morning we were happy with most of them, expect a few which were still abit clear. We decided to proceed anyway with plug making, adding chloramphenicol to them for a hour, then centrifuging down 3ml of all samples.
We then centifuged the others further down until all pellets appeared the same size and roughly the same size as the previous most successful plug making. While doing this we melted agarose in a waterbath slowly at 50C.
Once all pellets were appropriate sizes, we added cell suspension buffer, eluted the pellets and added agarose. We kept the agarose, cell suspension buffer and cells at 50C, and pipetted each sample into a mold. Producing 1 plug per sample.
Once solidified, we pushed each plug into a separate universal, added lysozyme, buffer and mutanolysin. This is now incubating at 37C until half 7/half 8pm. After which Mike will remove lysozyme, buffer and mutanolysin and add proteinase K and buffer, incubating it at 50C until tomorrow morning.
Unfortunatly we ran out of lysozyme and proteinase K buffers, so Mike had us make up our own from 1M tris HCL pH8 buffer, sodium dodeyl sulphate (SDS), 0.5M EDTA pH8 and pure water.
Tomorrow we will be washing the plugs and incubating them in SpeI. See you all tomorrow!
Hi all Catching up with last week the…
Hi all,
Catching up with last week, the plates i innoculated to try and trouble shoot the non growth issues i have been experiencing, showed that there is growth at each stage so at least i can rule out human error, yay! The strain of E.coli i am using (C2110) may be the issue as it is taking 6-7 hours to grow to an OD of 0.35, i would expect the growth to be in the region of 3 hours+. Leaving the plate with no growth on my desk to show mike the next day, miraculously i had fantastic growth overnight. I have already generated data for the plasmids pALA1029, pOG04 NJC, pOG04 DRW, pOG4003 and i am now starting with pOG4 and the Chloramphenicol resistant pNC0412B, fingers crossed all goes well.
Hey Everyone Today unfortunatly the 10 different strain…
Hey Everyone,
Today, unfortunatly the 10 different strain samples of propionibacterium acnes hadn’t grown enough to use yet, so we left them to grow longer all today and overnight.
We then removed the previously incubated old batch of plugs from SpeI enzyme, buffer and BSA. We incubated them all in 1 x wash buffer for 30mins at R.T. During that time we made up 1L of TAE 0.5x buffer, autoclaved it, allowed it to cool and used it to saturate the plugs for 1hr. We also used the buffer to make up a 1.4% gel, before cutting each of the 10 different strain propionibacterium acnes plugs from an old batch and placing them into wells. We also included a sample of the low range PFG marker. We then sealed the plugs using 1.4% agarose, we found a few problems whilst doing this. As we haven’t worked with as many plugs in the same gel at once before, one pipette tip (1ml) wasn’t enough to do all 11 wells. This was also made worst by how quickly the agarose cooled in the pipette creating bubbling and messy sealing. We plan to use a different tip for each sample next time, and work with slightly warmer agarose.
We then ran this gel for 18hrs, at 6V/cm, with 1-12 sec Switch interval. We also used the modified buffer flow method – which took a few times to balance effectively.
Tomorrow we will be hopefully starting plug production and viewing this latest gel. See you all then 🙂
Hello Today myself and Mike started by discussing…
Hello,
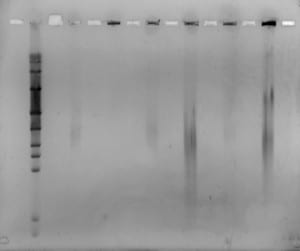
Today myself and Mike started by discussing the gel we ran last fri-sat. We produced pretty good results, as can be seen in the image attached. We could see much clearer bands, due to increased DNA, and slightly modified settings. 
We therefore decided to start produced new plugs using the 10 different strains of Propionibacterium acnes we tried to use before. Mike re cultured these strains yesterday, therefore we left them for today to grow further, until tomorrow.
We decided to run the old plugs we produced using these 10 different strains, to confirm lack of DNA. We removed the buffer the plugs were in, added 1ml per plug of SpeI 1x wash buffer, to saturate for an hour at R.T. We then added 0.3ml SpeI buffer, 3ul BSA and 7.5ul SpeI enzyme to each plug and incubated overnight, and through tomorrow at 37C. We did have some issues with the SpeI enzyme, due to a lack of enzyme from our original batch, which was 50,000u/ml. The new SpeI enzyme was 10,000u/ml and therefore required some modified calculations, taking into account increased incubation time.
Tomorrow we will be checking the growth of the 10 different strains of Propionibacterium acnes and then beginning new plug making, if appropriate. We will also be removing the old plugs from SpeI enzyme, buffer and BSA, and washing them in 1x wash buffer before storing them till weds.
Hey All Today i came in and filmed…
Hey All,
Today i came in and filmed my latest videoblog. I then removed the plugs from speI, buffer and BSA, and added 0.1x wash buffer for 30mins. While this was incubating, i autoclaved 1L of 0.5x TAE buffer, which i then used to incubate the plugs for an hour. I then prepared a 1.4% gel, with modified comb to create a large well. Once this had solidified i added one plug of the low range PFG marker, one whole plug of restriction enzyme treated plug in the large well, and finally one 1/3 of a restriction enzyme treated plug in one well. I then sealed the plugs, and ran the gel at 6v/cm for 15hrs, with 1-12sec switch interval ramping.
Mike will be in tomorrow to removed the gel, stain it and view. He will then save an image for me to observe with him on Monday 🙂 See you all then!
Hey Everyone Today was a loooong day Came…
Hey Everyone,
Today was a loooong day! Came in at 9am, and as the plugs were still fairly cloudy, Mike suggested we leave them abit longer to allow further lysis, and therefore make the plugs clearer. We made up a fresh solution of lysozyme, proteinase K and mutanolysin, and left the plugs to incubate further until 12pm.
I then began washing the plugs in 1x wash buffer, 1hr each four times. Once finished i washed the plugs in 0.1x wash buffer for an hour twice. I then removed the wash buffer and added speI buffer to allow the plugs to incubate for an hour. After this i added fresh buffer, SpeI enzyme and BSA. The plugs will incubate overnight at 37C.
I made appropriate modifications in amount of lysozyme, mutanolysin, SpeI buffer and enzyme etc. In order to compensate for an increase in DNA conc.
Tomorrow i will be filming my new videoblog, then incubating the plugs in 1x wash buffer, and in 0.5x TAE buffer, before setting up and running a new gel with our new modifications, that looked like they worked very well on weds.